Weekly Report
Overview v1.0
Article source: Date time:2020-06-29
Download:Overview v1.0
Overview of Influenza Surveillance in China
1. Reporting of notifiable diseases
According to the Law of the People's Republic of China on the Prevention and Control of Infectious Diseases, influenza is classified as a “C” category infectious disease and pandemic influenza H1N1 2009 is classified as a “B” category infectious disease. Therefore, when cases which meet the case definition of influenza and pandemic influenza H1N1 2009 are found, designated staff within CDC’s medical institutions should fill the case report forms for infectious disease within the specified time limit.
1.1 Report of influenza cases
According to the Influenza Diagnostic Criteria (WS 285-2008) implemented September 1, 2008, influenza cases can be divided into two types: clinical diagnosed cases and laboratory confirmed cases.
1.2 Report for pandemic influenza H1N1 2009 case
Designated staff in CDC’s medical institutions should record the case classification on a case report form for infectious disease. A case is classified as mild, severe or critical. Information regarding whether hospitalization also should be entered onto this form.
Specimen collection and laboratory detection should be performed as soon as possible for all hospital patients, including inpatients, outpatients and patients in emergency departments, who meet the definition for severe or critical cases of pandemic influenza H1N1 2009. On-line reports should be carried out at the same time. Clinical diagnosed cases should be corrected as laboratory confirmed cases if the detection results show positive. If within 24 hours after the laboratory confirmed case die or leave hospital, the hospital which received the case should fill in Case Report Form for Pandemic Influenza H1N1 2009 Severe Cases, and enter the information into “Pandemic Influenza H1N1 2009 Information Management System.” Positive specimens should be sent to a network laboratory for further analysis. For other specimens of ILI cases found, medical departments could send to network laboratories, but network laboratories are not necessary to detect on principal.
According to the Guideline for Pandemic Influenza H1N1 2009 Treatment (Third Version, 2009) published on October 12, 2009, definitions of pandemic influenza H1N1 2009 cases are as followed:
Clinical diagnosis of cases should be made by combined consideration of epidemiological history, clinical symptoms and virological detection. Early discovery and early diagnosis are the key for control, prevention and effective treatment.
1.2.1 Suspect case
Suspect case of pandemic influenza H1N1 2009 is defined as an individual with one or more of the following conditions:
(1) With ILI clinical symptoms, have close contacts with confirmed case of pandemic influenza H1N1 2009 in the viral shedding duration;
Close contacts refer to giving treatment, taking care of pandemic influenza H1N1 2009 confirmed cases in the shedding period without protection; touching their respiratory secretions, body fluids, etc.
(2) ILI clinical symptoms and has traveled to a region where pandemic influenza H1N1 2009 circulated (sustained human-to-human transmission and community-level outbreaks) in 7 days before onset.
(3) ILI clinical symptoms, nucleic acid detection positive for influenza A, but is unsubtyped.
For the above 3 condition, laboratory detection for pandemic influenza H1N1 2009 could be carried out.
1.2.2 Clinical diagnosed case
Only under the following conditions will a case be considered a clinical diagnosis case: in a pandemic influenza H1N1 2009 outbreak while some cases have been confirmed by laboratory detection, other cases with ILI symptoms but haven’t been confirmed by laboratory detection can be classified as clinical diagnosed cases.
Pandemic influenza H1N1 2009 outbreak is defined as a sudden increase of ILI cases in a unit or area, confirmed by laboratory detection as pandemic influenza H1N1 2009 outbreak.
Laboratory detection could be carried out for clinical diagnosed cases if condition permitted.
1.2.3 Laboratory confirmed case
Laboratory confirmed case of pandemic influenza H1N1 2009 is defined as an individual with ILI clinical symptoms and confirmed by one or more of the following tests:
-
Nucleic acid detection positive for pandemic influenza H1N1 2009 (real-time PCR or RT-PCR);
-
Pandemic influenza H1N1 2009 virus is isolated;
-
Four-fold rise in pandemic influenza H1N1 2009 virus specific neutralizing antibodies.
1.2.4 Severe case
Severe case is defined as an individual with one or more of the following conditions:
(1) High fever >3 days;
(2) Severe cough, sputum, bloody phlegm, or chest pain;
(3) Shortness of breath, difficulty breathing, cyanotic lips;
(4) Mental alteration: slow mental reaction, drowsiness, agitation, convulsions.
(5) Severe vomiting, diarrhea, dehydration;
(6) Imaging tests show signs of pneumonia;
(7) A sudden increase of CK, CK-MB;
(8) Marked worsening of underlying disease.
1.2.5 Critical case
Critical case is defined as an individual with one or more of the following conditions:
(1) Respiratory failure;
(2) Toxic shock;
(3) Failure of other organs;
(4) Other conditions requiring immediate admission to an intensive care unit.
Note: Before October 12, 2009, pandemic influenza H1N1 2009 cases only refers to suspected and laboratory confirmed cases in Guideline for Pandemic Influenza H1N1 2009 Treatment (Second Version, 2009).
2. Influenza Like Illness (ILI) sentinel surveillance
2.1 Definition of surveillance cases
ILI cases: fever (≥38℃), with cough or sore throat, and in the absence of other laboratory confirmed evidence.
2.2 Surveillance Period
All national ILI sentinel hospitals and network laboratories conduct surveillance throughout the whole year.
The influenza season starts from April to March in the following year.
Note: Before July, 2009, 13 provinces in northern area only carried out half-year surveillance; south area and 3 provinces in northern area (Liaoning, Tianjin, Gansu) carried out full year surveillance.
Northern area: Beijing, Hebei, Shanxi, Inner Mongolia, Liaoning, Jilin, Heilongjiang, Shandong, Tibet, Shanxi, Gansu, Qinghai, Ningxia and Xinjiang.
Southern area: Shanghai, Jiangsu, Zhejiang, Anhui, Fujian, Jiangxi, Hubei, Hunan, Guangdong, Guangxi, Hainan, Chongqing, Sichuan, Guizhou and Yunnan.
2.3 Arrangements of surveillance department
2.3.1 Urban general hospitals carry out ILI surveillance in internal medicine outpatient, internal medicine emergency, fever clinic and/or pediatric internal medicine outpatient and pediatric internal medicine emergency departments.
2.3.2 Urban pediatric hospitals carry out ILI surveillance in pediatric internal medicine outpatient, pediatric internal medicine emergency and/or fever clinics.
2.3.3 All internal medicine departments should be included in internal medicine outpatient for ILI surveillance.
2.3.4 All pediatric internal medicine departments should be included in pediatric internal medicine outpatient for ILI surveillance.
2.4 Report of an ILI case
2.4.1 Healthcare personnel of surveillance departments in sentinel hospitals should record ILI cases and total cases visited (denominator) for outpatient and emergency departments by age group. Departments responsible should collect these records everyday and enter the information into “Chinese Influenza Surveillance Information System” every Monday.
2.4.2 The source population of ILI cases and total cases visited outpatient and emergency should be the same.
2.4.3 Sentinel hospitals could arrange other proper departments to carry out surveillance by themselves.
2.5 Specimen collection of ILI case
2.5.1 Collection objectives: ILI cases onset within 3 days, without taking antiviral, ILI
Sentinel hospitals should collect 20-30 specimens every week. Specimens should be collected evenly during a week in order to obtain a more representative sample of the cases throughout the week. For example, sentinel hospitals could collect specimens once every two day (7-10 specimens once) or collect everyday (4-6 specimens once). Specimens should be kept at 4℃, and sent to network laboratories twice a week.
2.6 Laboratory Detection
Sentinel hospitals should send ILI specimens to their corresponding influenza surveillance network laboratory for detection. Network laboratory should handle specimens and carry out nucleic acid detection within 48 hours. Virus isolation and identification of pandemic influenza H1N1 2009 and season influenza should be performed in laboratories with the ability to carry out this detection while meeting the requirements of biosafety. All influenza virus strain should be sent to provincial CDCs for storage or identification. For the viruses which cannot be typed or subtyped and for positive specimens, these should be sent to Chinese National Influenza Center within 48 hours.
Note: Before the influenza surveillance network was enlarged in 2009, the exsisting 63 network laboratories carried out virus isolation directly after receiving specimens sent from sentinel hospitals.
3. Outbreak Surveillance
3.1 Definition of outbreak
A sudden increase of acute febrile respiratory illness cases in a unit or area, confirmed by laboratory detection as seasonal influenza or pandemic influenza H1N1 2009 outbreaks.
Relevant definition
Definition of acute febrile respiratory illness cases: fever (≥37.5℃), and with at least one of the following symptoms: cough, sore throat, nasal congestion, rhinorrhea.
3.2 Discovery and report of outbreaks
A cluster of acute febrile respiratory illnesses with more than 5 cases with evidence of epidemiological relationships within a unit or area within a week should be reported to county CDC by telephone or fax in 2 hours. Rural area should report to local township hospital.
Once outbreak report is received, county CDCs should organize a preliminary investigation to verify the outbreak, collect specimens of cases and send them to the qualified laboratory to detect for seasonal influenza or pandemic influenza H1N1 2009. The quantity of specimens should meet minimum requirement of laboratory detection.
If the outbreak is confirmed as seasonal influenza outbreak or pandemic influenza H1N1 2009 outbreak, county CDCs should fill in a Report Form for Emergent Public Health Events and Report Form for Seasonal Influenza and Pandemic Influenza H1N1 2009 Outbreak within 2 hours and report on-line through “Information System for Emergent Public Health Events.”
4. Construction of Chinese influenza surveillance network
As of 2005, the Chinese National Influenza Surveillance Network had already covered 31 provinces, autonomous regions and municipalities of mainland China, including 63 network laboratories and 197 national influenza surveillance sentinel hospitals. In March 2009, it was extended and 21 prefectural CDC laboratories joined in the network.
In April 2009, pandemic influenza H1N1 2009 outbreaks were reported. In order to better respond to these outbreaks, the Ministry of Health of China decided to greatly expand the Chinese National Influenza Surveillance Network. Since June 2009, an extensive surveillance network with 411 network laboratories and 556 national sentinel hospitals has been operating which not only covers all provinces, but also covers all prefectures and counties in mainland China. Therefore, China can now conduct pandemic influenza H1N1 2009 surveillance with improved capabilities which will lead to better understanding of the trends and variation of influenza viruses. This, in turn, will hopefully, significantly decrease the hazards of influenza and result in the protection of the physical and mental health of the public.
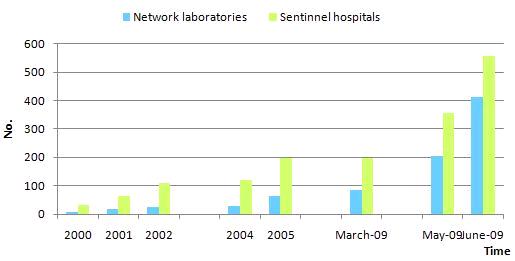
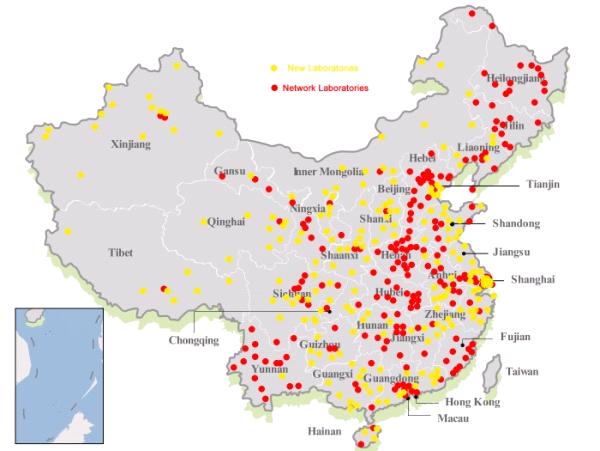
Figure 2 Geographic distribution of network laboratory and national sentinel hospitals, since June, 2009